Background and motivation
In the last years the development of new biosensors has increased significantly, especially in diagnostics, since it has been shown that an early diagnosis can change dramatically the development of a disease. In particular, in the third world the availability of biosensors for the most common diseases could save many lives. Unfortunately, the cost of biosensors and the lack of equipped centers and trained people are probably the hardest obstacles to the diffusion of adequate biosensors in these regions. The World Health Organization defined that diagnostics for developing countries should be defined as ASSURED: affordable, sensitive, specific, user-friendly, rapid and robust, equipment free and deliverable to end-users. Nanomaterials are bringing important advantages in the design of novel biosensing systems or improvements of the existing devices. Nanomaterial application in environmental monitoring (i.e. heavy metals), nanoparticles uses for DNA, proteins and even cells (i.e. cancer diagnostics) are showing a great potential in enhancing biosensor sensitivity, stability and in general improvement of the cost-efficiency of the developed devices. Although nanotechnology contains high level of integrated technologies and knowledge, it is also bringing simple sensing and biosensing concepts and technologies that are making possible the development of even more easy-to-use and efficient biosensors. Paper-based nanobiosensors are the excellent example of ASSURED devices developed as the result of the synergy between nanotechnology and biosensing technology. [1, 2, 3]
Nano-lantern on paper for smartphone-based ATP detection
 Several ATP kit assays are already commercially available but an user-friendly ATP biosensor characterized by low-cost, portability, and adequate sensitivity would be highly valuable for rapid and facile on site screening.
Several ATP kit assays are already commercially available but an user-friendly ATP biosensor characterized by low-cost, portability, and adequate sensitivity would be highly valuable for rapid and facile on site screening.
We developed a paper-based biosensor that detect 10−14 mol of ATP within 10 min and smartphone combined with a new lyophilisation strategy that enable to immobilize luciferase and reagents required for bioluminescent reaction directly on paper with higher reproducibility and enzyme stability than previously reported methods.
By exploiting paper as support a wax-printed nitrocellulose paper biosensor was developed using smartphone photo camera as light detector. [Biosens. Bioelectron.]
Signal enhancement on gold nanoparticle-based lateral flow tests using cellulose nanofibers
 The porosity of paper is a key factor regarding the sensitivity of the strips. While small pores will lead to higher sensitivity, the sample may have difficulties to flow, increasing the assay time and the probability of having a flow stop or membrane defects, further provoking low reproducibility. For this reason, we aim to decrease the pore size of the membrane only on the test area, where the recognition antibodies are dispensed. To accomplish this milestone, in this work we have included for the first time cellulose nanofibers (CNF) in the test area. CNF penetrate inside the pores of LFs nitrocellulose paper, compacting the pore size only in the test line, particularly near the surface of the strip. This modification retains the bioreceptors close to the surface of the strips, and thus further increasing the density of selectively attached gold nanoparticles in the top part of the membrane, in the test line area, only when the sample is positive. This effect boosts in average a 36.6% the sensitivity of the LFs. The optical measurements of the LFs were carried out with a mobile phone camera whose imaging resolution was improved by attaching microscopic lens on the camera objective. [Biosens. Bioelectron.]
The porosity of paper is a key factor regarding the sensitivity of the strips. While small pores will lead to higher sensitivity, the sample may have difficulties to flow, increasing the assay time and the probability of having a flow stop or membrane defects, further provoking low reproducibility. For this reason, we aim to decrease the pore size of the membrane only on the test area, where the recognition antibodies are dispensed. To accomplish this milestone, in this work we have included for the first time cellulose nanofibers (CNF) in the test area. CNF penetrate inside the pores of LFs nitrocellulose paper, compacting the pore size only in the test line, particularly near the surface of the strip. This modification retains the bioreceptors close to the surface of the strips, and thus further increasing the density of selectively attached gold nanoparticles in the top part of the membrane, in the test line area, only when the sample is positive. This effect boosts in average a 36.6% the sensitivity of the LFs. The optical measurements of the LFs were carried out with a mobile phone camera whose imaging resolution was improved by attaching microscopic lens on the camera objective. [Biosens. Bioelectron.]
Iridium oxide (IV) nanoparticle-based lateral flow immunoassay
 Looking for other advantageous nanomaterials, we propose for the first time the use of iridium oxide (IV) nanoparticles in lateral flow assays for the detection of human immunoglobulin as a model protein.
Looking for other advantageous nanomaterials, we propose for the first time the use of iridium oxide (IV) nanoparticles in lateral flow assays for the detection of human immunoglobulin as a model protein.
These nanoparticles can be easily prepared and conjugated with biomarkers. Their dark blue color gives a high contrast against the white background of the strips being in this way excellent labels. [Biosens. Bioelectron.]
Paper-based nanobiosensors architectures
We have demonstrated that very simple changes of the Au-NP based LFIA architecture, like increasing the size of both the conjugation and the sample pads, can improve its performance in terms of sensitivity of the assay. Flow speed simulations also corroborate the experimental achievements and can represent useful tools in designing novel lateral flow architectures. The proposed designs (Figure 1) can be easily applied in any type of LF strips without changing their fabrication method; moreover it is simple and cheap, fostering its use for point of care applications, even at the doctor office or in undeveloped countries. Studies using a different method of detection, based on the use of a camera , and the use of a wax printer, in order to define better the shape of the LFIA, have already started in our lab and they will enable decreasing the size of the detection pad enabling a further increment in the sensitivity of the device. [4]
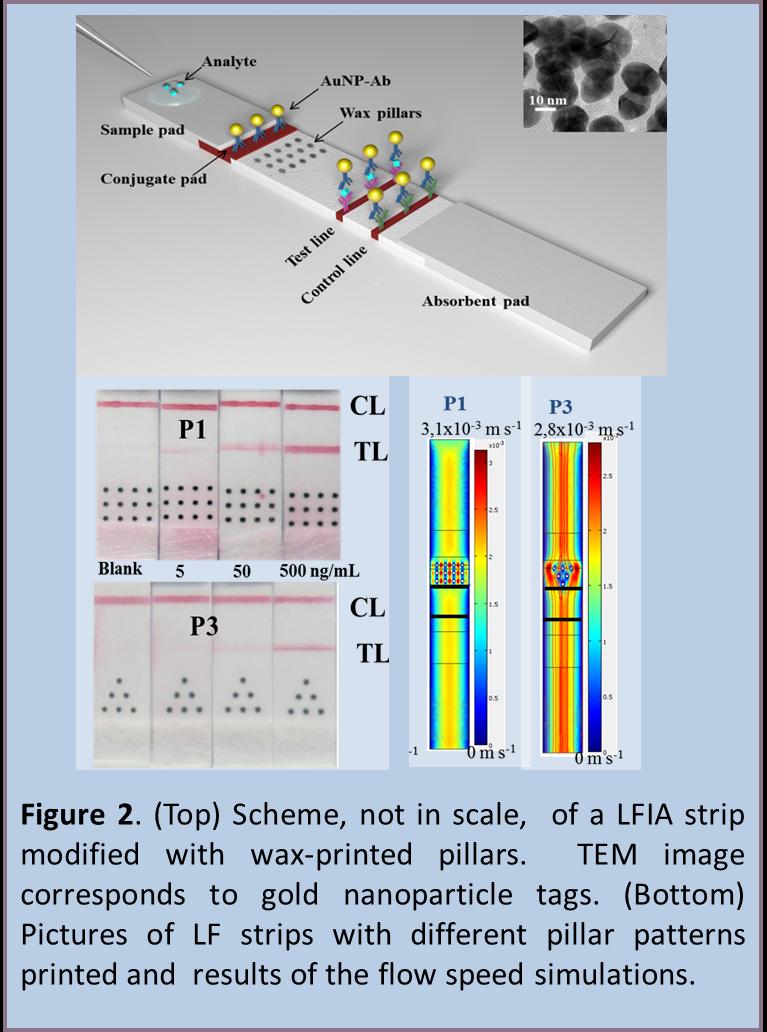
Use of wax-printed pillars as delay barriers of microfluidics
The most important part of a LFIA is the detection membrane which is made of cellulose nitrate or nitrocellulose (NC), a porous material where the capture reagents (e.g. antibodies) are immobilized due to a possible combination of electrostatic and hydrophobic forces. On the other hand, wax printing is a simple and low cost patterning technique based on the melting of solid wax printed onto porous substrate, which has been used for fabricating paper-based microfluidics in NC membrane. We propose a simple and facile alternative for improving the LFIA sensitivity based on the use of delay hydrophobic barriers fabricated by wax-printing. Several wax pillars patterns are printed onto nitrocellulose membrane in order to produce delays as well as pseudo turbulences into the microcapillary flow (Figure 2). The effect of the proposed wax pillar modified devices are also mathematically simulated corroborating the experimental results obtained for the different patterns tested afterwards for detection of HIgG as model protein in a gold nanoparticle-based LFIA. The effect of the introduction of such wax-printed pillars is the sensitivity improvement of almost 3-folds in comparison to a conventional free-barrier LFIA. [5]
Novel labelling strategies for paper-based lateral flow devices
The enhancement of the sensitivity of lateral flow immunoanalysis based on paper (Paper-LFIA) platforms is strongly related to the sensitivity of the labels. The development of a Paper-LFIAs based on the use of AuNPs not only as labels but also as carriers of enzymatic labels is performed (Figure 3). AuNPs produce red bands at the detection and control lines of the LFIA when acting as direct labels; but if they are coupled with an antibody modified with HRP they can also act as carriers. 3,3′,5,5′-Tetramethylbenzidine (TMB); 3-Amino-9-ethylcarbazole (AEC); 3,3′-Diaminobenzidine tetrahydrochloride (DAB) with Metal Enhancer as substrates of the HRP are evaluated since they produce insoluble chromogens which cannot be moved by the flow, concentrating the color at the lines. The developed LFIAs offer two different detection alternatives: one produced just by the red color of the AuNPs and one more sensitive produced by the substrate of the HRP achieving an ‘on-demand’ tuning of the biosensing performance. Its application for protein detection, after related optimizations, could open the way to several uses with interest in diagnostics, safety and security between other fields. [6]
Lab-in-a-syringe using gold nanoparticles
In addition to their low sensitivity, two major limitations of LFAs are related to the requirement of samples poured directly onto the (exposed) sample pad, which can be dangerous, especially for handling of hazardous samples, and to the fact that they can only handle small volumes (typically 200 microliters) of sample per analysis. Given the aforementioned context, we propose a new, paper and gold nanoparticle-based lab-in-a-syringe (LIS) device. It employs a simple vertical-flow immunoassay (VFIA) that operates simultaneously to sampling. By inserting the conjugate pad and detection pad inside plastic cartridges that can be connected to a syringe, we are able to minimize handling of the sample by the operator and expand the volume of sample that can be analyzed (up to 5 milliliters), thereby making the device safer and more sensitive than similar tools (Figure 4). Like most other paper-based diagnostic devices, our new LIS is easy to use and fast, providing a read out in less than 10 minutes. We demonstrate its applicability for the detection of PSA in real human urine samples at clinically relevant concentrations. Beyond this clinical proof-of-concept application, our LIS has enormous potential for applications in fields such as food control, safety and security, and environmental analysis.[7]
Triple lines gold nanoparticle-based lateral flow for enhanced DNA detection
The principles of an indirect ELISA assay are also applied in a novel design for signal enhancement in LFAs. Secondary antibodies are included in the conjugate pad and applied for the detection of amplified Leishmania infantum DNA extracted from dog blood. The polyclonal nature of such antibodies allows their multiple connections with primary ones, giving rise to the enhancement of the AuNP signal in the test line and consequently the sensitivity of the assay is highly increased. The use of labelled primers in the DNA amplification procedure allows to obtain double labelled (FITC/biotin) amplified DNA products that can be detected in a LFA., allowing to detect up to 0.038 parasites per DNA amplification reaction (1 parasite/100mL of DNA) (Figure 5).
Furthermore, an additional control, the so- called endogen control, is included in a second test line so as to avoid false negatives. This approach is successfully implemented, without losing the efficiency of the signal enhancement. The proposed enhancement strategy is a versatile and universal methodology that can be applied for any LFA design. It also has the advantage of the fact that the specific antibody against the analyte does not need to be directly labelled, representing clear advantages in terms of technology cost. [8]
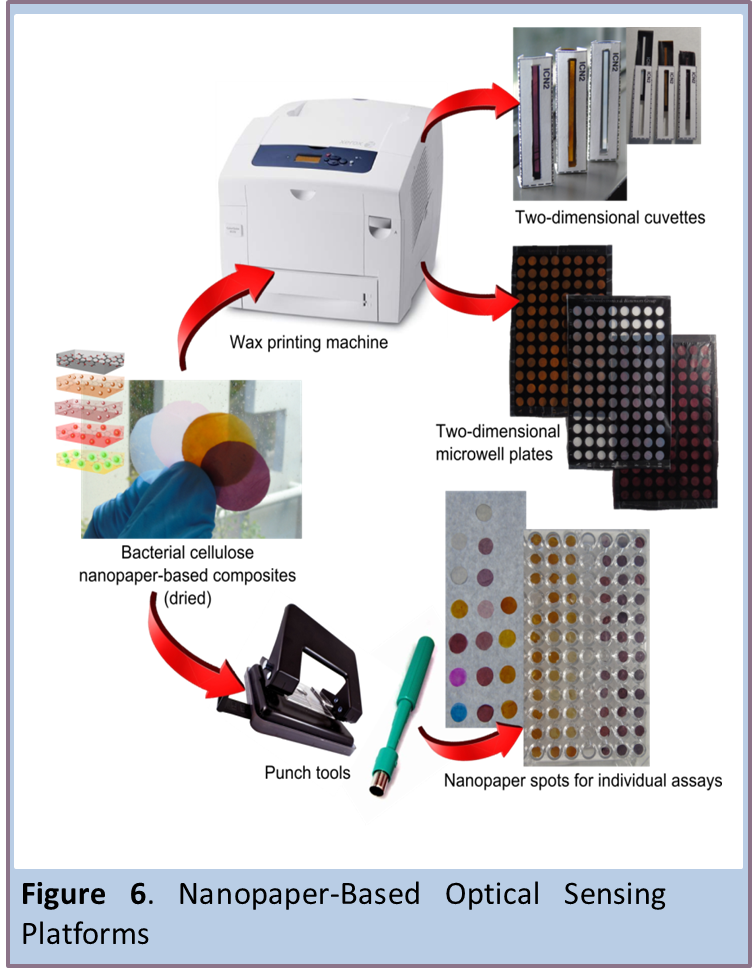
Nanopaper-Based Optical Sensing Platforms
Bacterial cellulose nanopaper (BC) is a multifunctional material known for numerous desirable properties: sustainability, biocompatibility, biodegradability, optical transparency, thermal properties, flexibility, high mechanical strength, hydrophilicity, high porosity, broad chemical-modification capabilities and high surface area. Herein, we report various nanopaper-based optical sensing platforms and describe how they can be tuned, using nanomaterials, to exhibit plasmonic or photoluminescent properties that can be exploited for sensing applications (Figure 6). We also describe several nanopaper configurations, including cuvettes, plates and spots that we printed or punched on BC. The platforms include a colorimetric-based sensor based on nanopaper containing embedded silver and gold nanoparticles; a photoluminescent-based sensor, comprising CdSe@ZnS quantum dots conjugated to nanopaper; and a potential up-conversion sensing platform constructed from nanopaper functionalized with NaYF4:Yb3+@Er3+&SiO2 nanoparticles. We have explored modulation of the plasmonic or photoluminescent properties of these platforms using various model biologically relevant analytes. Moreover, we prove that BC is and advantageous preconcentration platform that facilitates the analysis of small volumes of optically active materials (∼4 μL). We are confident that these platforms will pave the way to optical (bio)sensors or theranostic devices that are simple, transparent, flexible, disposable, lightweight, miniaturized and perhaps wearable. [9, 10]
Visual Detection of Volatile Compounds in a Piece of Plasmonic Nanopaper
We also discovered that the population density and size of silver nanoparticles embedded within bacterial cellulose can be readily modulated by volatile compounds exposure, leading to a simple naked-eye detection of a hazardous corrosive vapour (ammonia) or a mixture of volatile compounds released during food spoilage using a piece of plasmonic nanopaper (Figure 7). In fact, such a plasmonic nanopaper exhibits a change in colour from amber to light amber upon ammonia vapour exposure and from amber to a grey or taupe colour upon fish or meat spoilage exposure. [11]
Screening device with smart-phone readout
In our group it has been reported an easy-to-use, low cost, and disposable paper-based sensing device for rapid chemical screening with a smartphone readout. The device comprises luminescent graphene quantum dots (GQDs) sensing probes embedded into a nitrocellulose matrix where the resonance energy transfer phenomenon is applied as the sensing mechanism (Figure 8A). The GQDs probes were synthesized from citric acid by a pyrolysis procedure, further physisorbed and confined into small wax-traced spots on the nitrocellulose substrate. The GQDs were excited by an UV LED powered by a smartphone which is used as both energy source and imaging capture. The LED was contained within a 3D-printed dark chamber that isolates the paper platform from external light fluctuations leading to highly reproducible data (Figure 8B). The cellulose-based device was proven as a promising screening tool for phenols and polyphenols in environmental and food samples, respectively. It opens up new opportunities for simple and fast screening of organic compounds and offers numerous possibilities for versatile applications. It can be especially useful in remote settings where sophisticated instrumentation is not always available.[12]
Selected references:
1. Claudio Parolo, Arben Merkoçi, “Paper based nanobiosensors for diagnostics”, Chem. Soc. Rev., 42, 2013, 450-457.
2. Adaris M. López-Marzo, Arben Merkoçi, “Paper-based sensors and assays: a success of the engineering design and the convergence of knowledge areas”, Lab Chip, 16, 2016, 3150-3176.
3. Daniel Quesada-González, Arben Merkoçi, “Nanoparticle-based lateral flow biosensors”, Biosensors and Bioelectronics, 73, 2015, 47–63.
4. Claudio Parolo, Alfredo de la Escosura-Muñiz, Arben Merkoçi, “Simple paper architecture modifications lead to enhanced sensitivity in nanoparticle based lateral flow immunoassay”, Lab Chip, 13, 2013, 386-390.
5. Lourdes Rivas, Mariana Medina, Alfredo de la Escosura-Muñiz and Arben Merkoçi, «Improving sensitivity of gold nanoparticles-based lateral flow assays by using wax-printed pillars as delay barriers of microfluidics«, Lab Chip, 14, 2014, 4406-4414.
6. Claudio Parolo, Alfredo de la Escosura-Muñiz, Arben Merkoçi, “Enhanced lateral flow immunoassay using gold nanoparticles loaded with enzymes”, Biosens. Bioelectron., 40, 2013, 412-416.
7. Gisele Elias Nunes-Pauli, Alfredo de la Escosura-Muñiz, Claudio Parolo, Ivan H. Bechtold, Arben Merkoçi, «Lab-in-a-syringe using gold nanoparticles for rapid immunosensing of protein biomarkers», Lab Chip, 15, 2015, 399-405.
8. Lourdes Rivas, Alfredo de la Escosura-Muñiz, Lorena Serrano, Laura Altet, Olga Francino, Armand Sánchez, Arben Merkoçi. “Triple lines gold nanoparticle-based lateral flow for enhanced and simultaneous Leishmania DNA detection and endogenous control”, Nano Research 2015, Volume 8 (11), 3704-3714.
9. Eden Morales-Narváez, Hamed Golmohammadi, Tina Naghdi, Hossein Yousefi, Uliana Kostiv, Daniel Horak, Nahid Pourreza, Arben Merkoçi.“Nanopaper as an optical sensing platform“, ACS Nano, 9, 2015, 7296-7305.
10. Hamed Golmohammadi, Eden Morales-Narváez, Tina Naghdi, Arben Merkoçi. “Nanocellulose in Sensing and Biosensing”, Chem. Mater., 2017, 29 (13), 5426–5446.
11. B. Heli,E. Morales-Narváez, H. Golmohammadi, A. Ajji and A. Merkoçi, “Modulation of population density and size of silver nanoparticles embedded in bacterial cellulose via ammonia exposure: visual detection of volatile compounds in a piece of plasmonic nanopaper“, Nanoscale, 2016, 8, 7984.
12. Jahir Orozco, Ruslan Álvarez Diduk, Arben Merkoçi, “Paper strip-embedded graphene quantum dots: a screening device with a smartphone readout”, Sci. Rep., 2017, 7 (976).