Background and motivation
The coupling of enzymes as biocatalytic amplifying labels is a generated paradigm in developing bioelectronic sensing devices. The biocatalytic generation of a redox product upon binding of the label to the recognition event, the incorporation of redox mediators into DNA or other biomolecules assemblies that activate bioelectrocatalytic transformations, or the use of enzyme labels that yield an insoluble product on electrode surfaces has been extensively used to amplify biorecognition events. Due to several problems associated with these techniques and the fast development in nanotechnology, nanoparticles (NPs) assisted signal enhancement for DNA, protein, cells and enzyme based biosensors has been greatly developed in the last decade. The recent developments of nanotechnology lead the scientists to fabricate and analyze catalysts at the nanoscale. These nanostructured materials are usually high-surface-area metals or semiconductors in form of NPs with excellent catalytic properties due to the high ratio of surface atoms with free valences to the cluster of total atoms. The catalysis takes place on the active surface sites of metal clusters in a similar mechanism as the conventional heterogeneous catalysis and in general this is a process that occurs at the molecular or atomic level independently of the catalyst dimensions. Employing NPs in electroanalysis can induce more sensitive and selective sensors as well as more cost effective and portable systems. Their application as catalysts in electroanalytical systems, can decrease overpotentials of many important redox species, inducing discrimination between different electroactive analytes, and also allow the occurrence and reversibility of some redox reactions, which are irreversible at common modified electrodes. The catalytic effect is related with the enhancement of electron transfer between the electrode surface and the species in solution, by enhancement of mass transport or also by the NPs high surface energy that allows the preferred adsorption of some species that by this way suffer a change in their overpotentials. In addition to catalytic properties nanoparticles (ex. gold nanoparticles) can be offered as carriers of other electro/optoactive compounds (ex. enzymes) being in this mode very interesting building blocks for various biosensing technologies. [1,2,3,4]
Gold nanoparticles as carriers of enzymes
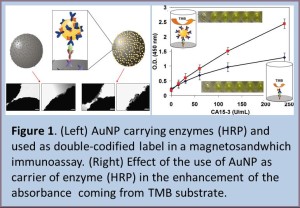
A novel double-codified nanolabel (DC-AuNP) based on gold nanoparticle (AuNP) modified with anti-human IgG peroxidase (HRP)-conjugated antibody is reported. [5] It represents a simple assay (Figure 1, Left) that allows enhanced spectrophotometric and electrochemical detection of antigen human IgG as a model protein. The method takes advantage of two properties of the DC-AuNP label: first, the HRP label activity toward the OPD chromogen that can be related to the analyte concentration and measured spectrophotometrically; second, the intrinsic lectrochemical properties of the gold nanoparticle labels that being proportional to the protein concentration can be directly quantified by stripping voltammetry. Beside these two main direct determinations of human IgG, a secondary indirect detection was also applicable to this system, exploiting the high molar absorptivity of gold colloids, by which, the color intensity of their solution was proportional to the concentration of the antigen used in the assay. Paramagnetic beads were used as supporting material to immobilize the sandwich-type immunocomplexes resulting in incubation and washing times shorter than those typically needed in classical ELISA tests by means of a rapid magnetic separation of the unbound components. A built-in magnet graphite-epoxy-composite electrode allowed a sensibly enhanced adsorption and electrochemical quantification of the specifically captured AuNPs. The used DC-AuNP label showed an excellent specificity/ selectivity, as a matter of fact using a different antigen (goat IgG) a minimal nonspecific electrochemical or spectrophotometric signal was measured. The detection limits for this novel double-codified nanoparticle-based assay were 52 and 260 pg of human IgG/mL for the spectrophotometric (HRP-based) and electrochemical (AuNP-based) detections, respectively, much lower than those typically achieved by ELISA tests.
Following the same idea we also demonstrate the application of AuNPs as a multienzyme carrier to the enzyme based immunoassay for the detection of CA15-3 breast cancer biomarker. [6] The use of AuNPs allows the attachment of a multiple enzyme system which can generate an amplified optical signal, while keeping low background signals. The use of AuNPs as signal enhancers not only resulted in better performances (the sensitivity is doubled compared to the test where no AuNPs were used) (Figure 1, right) but also shortened the incubation time for the color developer TMB (5 min compared to 30 min for the classical procedure).
Gold nanoparticles as electrocatalysts
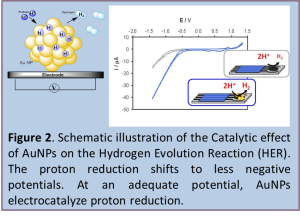
In the wide range of nanomaterials, gold nanoparticles (AuNPs) grab a lot of attention as they have been applied in numerous nanobiotechnological applications. Following the selective electrocatalytic reduction of silver ions onto the surface of gold nanoparticles (AuNP) [7] we have moved toward novel catalytic effects. Bulk gold is considered an inert material toward redox processes due to the repulsion between the filled d-states of gold and molecular orbitals of molecules like O2 or H2, but small AuNPs contain a large number of coordinative unsaturated atoms in edge positions. The quantum effects related with shape and size of AuNPs originated by d band electrons of the surface which are shifted towards the Fermi-level, promote the ability to interact in electrocatalytic reactions. All these features allow the occurrence of adsorption phenomena with catalytic properties, and places AuNPs in the palette of materials with potential interest to be used in electrocatalyzed reactions. Furthermore they exhibit good biological compatibility and excellent conductivity that highlights them for biosensor applications. We make use of the advantageous characteristics of screen-printed carbon electrodes (SPCEs) in terms of low cost, miniaturization possibilities, sample consuming and the wide working potential range in the Hydrogen Evolution Reaction (HER) in presence of AuNPs. [8,9,10,11,12,13,14,15] In addition we combine all the mentioned advantages with the relative high hydrogen overpotential and low background currents for the detection of AuNPs using SPCEs (Figure 2). This is based on the excellent electroactive properties of AuNPs to catalyze HER in acidic media which is measured by recording the current generated in the simple and efficient chronoamperometric mode.
In another work, a novel methodology for the isothermal amplification of Leishmania DNA using labeled primers combined with the advantages of magnetic purification/preconcentration and the use of gold nanoparticle (AuNP) tags for the sensitive electrochemical detection of such amplified DNA was developed. [15] (Figure 3) Primers labeled with AuNPs and magnetic beads (MBs) are used for the first time for the isothermal amplification reaction, being the amplified product ready for the electrochemical detection. The electrocatalytic activity of the AuNP tags toward the hydrogen evolution reaction allows the rapid quantification of the DNA on screen-printed carbon electrodes. Amplified products from the blood of dogs with Leishmania (positive samples) are discriminated from those of healthy dogs (blank samples). Quantitative studies demonstrate that the optimized method allows us to detect less than one parasite per microliter of blood (8 × 10−3 parasites in the isothermal amplification reaction). This pioneering approach is much more sensitive than traditional methods based on real-time polymerase chain reaction (PCR), and is also more rapid, cheap, and user-friendly.we are looking now for novel and high sensitive electrocatalytic materials to be used in biosensing technology either as labels for DNA or immunosensing or as modifiers of transducing platforms. [16,17]
Iridium Oxide nanoparticles as electrocatalysts
The use of high acidic solutions necessary for the detection of NPs such as AuNPs, in addition to inherent security risks, represents an additional step which not only increases the analysis time but also is a crucial limitation in case of really integrated sensing systems, such as those based on lab-on-a-chip or lateral-flow platforms. Hydrogen bubbles formed during hydrogen gas evolution is another inconvenience for the integration of HER based biosensing systems in microfluidics platforms. For these reasons, there is a demand for novel NP tags easy to be detected in the same medium of the immunoreaction, often saline buffers at neutral pH. In this context NPs able to catalyze water oxidation reaction (WOR) would be ideal candidates for this purpose. We demonstrate that Iridium oxide nanoparticles (IrO2 NPs) synthesized by our group are excellent candidates for their use as novel tags for biosensing taking advantage of their electrocatalytic activity towards WOR at neutral pH. The chronoamperometric current recorded at a fixed potential of +1.3 V constitutes the analytical signal allowing the quantification of IrO2 NPs at nM levels (Figure 4). Moreover, the UV-Vis absorption band observed at 590 nm (deep blue color) of the IrO2 NPs suspension make these NPs excellent candidates for dual electrochemical/optical detection systems in future lateral flow biodetection platforms. [18]
Iridium Oxide nanoparticles as enhancers of electrode conductivity
It is well-known that nanomaterials represent a powerful tool for modifying electrode surfaces thanks to their high surface-to-volume ratio and good conductivity, which make them useful for proposing novel electrochemical biosensors or greatly improving the existing ones. Iridium-oxide-based materials (in films and nanoparticle form) are attractive because of their catalytic activity, biocompatibility, and outstanding chemical and thermal stability, which have been used for applications in pH sensors, neural stimulation, and environmental biosensors. In this context, we have developed an OTA aptasensor that takes advantage of electropolymerized thionine films onto SPCE and iridium oxide nanoparticles (IrO2NPs). Electrochemical impedance spectroscopy is used to monitor each step in the aptasensor development and also to detect OTA (Figure 5). The charge transfer resistance increases proportionally to the concentration of OTA in a linear range of 0.01− 100 nM. This system shows also good reproducibility, sensitivity, and selectivity. To the best of our knowledge, this sensitive aptasensor exhibits one of the lowest limits of detection (LOD = 14 pM) reported so far for electrochemical detection of OTA. [19]
PCR amplified Leishmania infantum DNA has also been detected in a very sensitive label-free approach using specific ssDNA probes immobilized on IrO2NPs modified screen-printed carbon electrodes [20] (Figure 6).This system is based on a screen-printed carbon electrode modified with the thionine layer and iridium oxide nanoparticles (IrO2 NP). An aminated oligonucleotide probe is immobilized on the IrO2 NP/polythionine modified electrode and ethanolamine was used as a blocking agent. Different diluted PCR amplified DNA samples have been detected. The selectivity and reproducibility of this system are studied and the system was highly reproducible with RSD ≈ 15% and sensitive enough while using 2% of ethanolamine during the blocking step employed for genosensor preparation.
We also designed a methimazole (MT) biosensor based on a nanocomposite of magnetic nanoparticles (MNPs) functionalized with iridium oxide nanoparticles (IrOx NPs) and tyrosinase (Tyr) immobilized onto screen printed electrode (SPE) by using a permanent magnet [21] (Figure 7). This system is evaluated in batch mode via chelating copper at the active site of tyrosinase and in flow mode by thioquinone formation. The MT detection in flow mode is achieved using a hybrid polydimethylsiloxane/polyester amperometric lab-on-a-chip (LOC) microsystem with an integrated SPE. Both systems are very sensitive with low limit of detection (LOD): 0.006 μM and 0.004 μM for batch and flow modes, respectively. Nevertheless, the flow mode has advantages such as its reusability, automation, low sample volume (6 μL), and fast response (20 s). Optimization and validation parameters such as enzyme-substrate amount, flow rate, inhibition conditions, repeatability and reproducibility of the biosensor have been performed. The proposed methods have been applied in MT detection in spiked human serum and pharmaceutical dosage forms.
In additon, a novel biosensor based on electrochemically reduced graphene oxide and iridium oxide nanoparticles for the detection of angiotensin-converting enzyme inhibitor drug, captopril, is presented [22] (Figure 8). For the preparation of the biosensor, tyrosinase is immobilized onto screen printed electrode by using 1-Ethyl-3-(3-dimethylaminopropyl)-carbodiimide and N-Hydroxysuccinimide coupling reagents, in electrochemically reduced graphene oxide and iridium oxide nanoparticles matrix. Biosensor response is characterized towards catechol, in terms of graphene oxide concentration, number of cycles to reduce graphene oxide, volume of iridium oxide nanoparticles and tyrosinase solution. The designed biosensor is used to inhibit tyrosinase activity by Captopril, which is generally used to treat congestive heart failure. It is an angiotensin-converting enzyme inhibitor that operates via chelating copper at the active site of tyrosinase and thioquinone formation. The captopril detections using both inhibition ways are very sensitive with low limits of detection: 0.019 µM and 0.008 µM for chelating copper at the active site of tyrosinase and thioquinone formation, respectively. The proposed methods have been successfully applied in captopril determination in spiked human serum and pharmaceutical dosage forms with acceptable recovery values.
Copper oxide nanoparticles as non-enzymatic biosensing element
Nanotechnology offers the possibility of designing and producing reliable non-enzymatic sensors with a fast response, overcoming limitations related to low stability and need for complex purification procedures of the biological recognition elements normally used as receptors. These materials artificially mimic biomolecules through their catalytic properties. Great interest has recently been shown in copper oxide (CuO) due to its diverse properties as an antioxidant, conductor and catalyst. CuO NPs have been previously used for non-enzymatic electrochemical sensing of glucose and other molecules. In this context, we have proposed the use of CuO NPs in novel non-enzymatic electrochemical systems (Figure 9), taking advantage of the interactions of CuO NPs with toxic compounds that in turn generate electrochemical signals related to the concentration of pollutants. [23]
Nanowires / nanotubes
The use of nanomaterials such as Au, Pt–Pb, Ag, and Cu nanoparticles, Pt–Pb and Au nanowires, mesoporous Pt, three-dimensional dendritic Pt nanostructures, nanoporous Au, CuO nanowire arrays, various alloys such as the nanoporous Pt-Ir alloy, Pb-Pt, boron-doped nanocrystalline diamond thin film and carbon nanotubes (CNTs) are showing a great interest for the development of reliable nonenzymatic / enzyme ‘mimick’ sensors.
Development of nonenzymatic sensors to solve problems related to stability is of great importance. Nanostructured electrodes possess a very large surface to area activation ratio, favouring kinetically controlled reactions like electrocatalytic oxidation of glucose more than diffusion controlled reactions expecting a high sensitivity toward glucose detection.For this reason we proposed the use of a bimetallic platinum/gold nanowire (Au-Pt NW) as a free-enzyme electrocatalyst for glucose. (Figure 10) This was achieved through Au-Pt NW integration on the working electrode of a screen printed electrode (SPE) followed by Chronoimpedance technique (CIT) measurements performed for the first time in an integrated and miniaturized three electrode system. The use of an iridium oxide (IrOx) thin layer electrodeposited onto the working electrode surface was necessary to reduce the electrode-electrolyte interface impedance (EEIZ) for CIT measurements. The resulting nonenzymatic glucose sensors thanks to the use of the bimetallic nanowires exhibited highly sensitive response toward glucose detection, showing a linear response up to 140µM. [24]
Solid-State Ag/AgCl Pseudoreference Electrode
One of the most important components of electrochemical sensors is the reference electrode. A miniaturized, disposable and low cost Ag/AgCl pseudo-reference electrode based on inkjet printing has been developed in our group [25] (Figure 11). Silver ink was printed and chlorinated with bleach solution. The reference electrodes obtained in this work showed a good reproducibility and stability during at least 30 minutes continuous measurement and even after 30 days stored without specials carefulness. Moreover, the strategy used in this work can be useful for large scale production of solid-state Ag/AgCl pseudo-reference electrode with different designs and sizes, facilitating the coupling with different electrical/electrochemical micro-sensors and biosensors.
Nowadays, nanomaterials are considered a pivotal tool for different fields such as textiles, energy, environment, electronics, photonics, food, agriculture, biomedicine and health care. This is due to their advantageous properties coming from their high surface area, among other physicochemical properties, compared to their respective bulk forms. Nanomaterials, while used in (bio)detection systems, have shown to be extremely valuable to improve the analytical performance of conventional/laboratory methods and move forward biosensing technology. The usage of nanomaterials has been widely spread over the last few years mainly thanks to the great advantages that they offer in the development of conceptually new biosensors or improving the existing ones. [26]
Selected references
- Alfredo de la Escosura-Muñiz, Adriano Ambrosi, Arben Merkoçi, “Electrochemical analysis with nanoparticle-based biosystems”, Trends in Analytical Chemistry, 27, 2008, 568-584.
- Alfredo de la Escosura-Muñiz, Arben Merkoçi, “Electrochemical detection of proteins using nanoparticles: applications to diagnostics”, Expert Opinion on Medical Diagnostics, 4(1), 2010, 21-37.
- Alfredo de la Escosura-Muñiz, Claudio Parolo, Arben Merkoçi, “Immunosensing using nanoparticles”, Materials Today, 13, 2010, 24-34.
- Alfredo de la Escosura-Muñiz, Arben Merkoçi. “Nanoparticles for proteins and cells detection. Novel tools for clinical diagnostics”, G.I.T. Laboratory Journal, 1-2, 2012, 21-23
- Adriano Ambrosi, Maria Teresa Castañeda, Anthony J. Killard, Malcolm R. Smyth, Salvador Alegret, Arben Merkoçi, “Double-Codified Gold Nanolabels for Enhanced Immunoanalysis”, Anal. Chem., 79, 2007, 5232-5240.
- Adriano Ambrosi, Federico Airò, Arben Merkoçi, “Enhanced Gold Nanoparticle based ELISA for Breast Cancer Biomarker”, Anal. Chem., 82, 2010, 1151-1156.
- Alfredo de la Escosura-Muñiz, Marisa Maltez-da Costa, Arben Merkoçi, “Controlling the electrochemical deposition of silver onto gold nanoparticles: Reducing interferences and increasing the sensitivity of magnetoimmuno assays”, Biosens. Bioelectron., 24, 2009, 2475-2482
- Marisa Maltez-da Costa, Alfredo de la Escosura-Muñiz, Arben Merkoçi, “Electrochemical quantification of gold nanoparticles based on their catalytic properties on hydrogen formation: application in magneto immunoassays”, Electrochem. Commun., 12, 2010, 1501-1504.
- Alfredo de la Escosura-Muñiz, Christian Sánchez-Espinel, Belén Díaz-Feitas, África González-Fernández, Marisa Maltez-da Costa, Arben Merkoçi, “Rapid identification and quantification of tumor cells using an electrocatalytic method based on gold nanoparticles”, Anal. Chem., 81, 2009, 10268-10274.
- Alfredo de la Escosura-Muñiz, Marisa Maltez-da Costa, Christian Sánchez Espinel, Belén Díaz-Freitas, Jonathan Fernández-Suárez, África González-Fernández, Arben Merkoçi, “Gold nanoparticle-based electrochemical magnetoimmunosensor for rapid detection of anti-hepatitis B virus antibodies in human serum”, Biosens. Bioelectron., 26, 2010, 1710-1714.
- Marisa Maltez-da Costa, Alfredo de la Escosura-Muñiz, Carme Nogués, Lleonard Barrios, Elena Ibáñez, Arben MerkoçI, “Detection of circulating cancer cells using electrocatalytic gold nanoparticles”, Small, 8(23), 2012, 3605-3612.
- Marisa Maltez-da Costa, Alfredo de la Escosura-Muñiz, Carme Nogués, Lleonard Barrios, Elena Ibáñez, Arben Merkoçi, “Simple monitoring of cancer cells using nanoparticles”, Nano Letters, 12, 2012, 4164-4171.
- Alfredo de la Escosura-Muñiz, Zdenek Plichta, Daniel Horák, Arben Merkoçi, “Alzheimer′s disease biomarkers detection in human samples by efficient capturing through porous magnetic microspheres and labelling with electrocatalytic gold nanoparticles”. Biosens. Bioelectron., 67 , 2015, 162–169.
- Abdel-Rahim Hussein Abdel-Azzem Hassan, Alfredo de la Escosura-Muñiz, Arben Merkoçi, “Highly sensitive and rapid determination of Escherichia coli O157:H7 in minced beef and water using electrocatalytic gold nanoparticle tags”. Biosens. Bioelectron.,67, 2015, 511–515.
- Alfredo de la Escosura-Muñiz, Luis Baptista-Pires, Lorena Serrano, Laura Altet, Olga Francino, Armand Sánchez and Arben Merkoçi. “Magnetic Bead/Gold Nanoparticle Double-Labeled Primers for Electrochemical Detection of Isothermal Amplified Leishmania DNA”. Small, 12(2), 2016, 205–213.
- Alfredo de la Escosura-Muñiz, Claudio Parolo, Arben Merkoçi, “Immunosensing using nanoparticles”, Materials Today, 13, 2010, 24-34.
- Marisa Maltez-da Costa, Alfredo de la Escosura-Muñiz, Arben Merkoçi. “Nanoparticles- induced catalysis for electrochemical DNA biosensors”, Chapter at book: Electrochemical DNA Biosensors, edited by M. Oszoz, published by Pan Stanford. Year: 2012, Chapter: 5, Pages: 141-162 ISBN 978-981-4241-77-9 (Hardcover); ISBN 978-981-4303-98-9 (eBook).
- Lourdes Rivas, Alfredo de la Escosura-Muñiz, Josefina Pons, Arben Merkoçi; “Alzheimer disease biomarker detection through electrocatalytic water oxidation induced by Iridium Oxide nanoparticles”,Electroanalysis, 26(6), 2014, 1287-1294.
- Carmen C. Mayorga-Martinez, Maria Guix, Rossana E. Madrid, Arben Merkoçi, “Bimetallic nanowires as electrocatalyst for nonenzymatic real time impedancimetric detection of dlucose”. Chem. Comm., 48, 2012, 1686-1688.
- Carmen C. Mayorga-Martinez, Alejandro Chamorro-García, Lorena Serrano, Lourdes Rivas, Daniel Quesada-Gonzalez, Laura Altet, Olga Francino, Armand Sánchez ,Arben Merkoçi. “An iridium oxide nanoparticle and polythionine thin film based platform for sensitive Leishmania DNA detection“.J. Mater. Chem. B, 3, 2015, 5166-5171.
- Sevinc Kurbanoglu, Carmen C. Mayorga-Martinez, Mariana Medina-Sánchez, Lourdes Rivas, Sibel A. Ozkan, Arben Merkoçi, “Antithyroid drug detection using an enzyme cascade blocking in a nanoparticle‐based lab‐on‐a‐chip system”. Biosens. Bioelectron., 67, 2015, 670–676.
- Sevinc Kurbanoglu, Lourdes Rivas, Sibel A. Ozkan, Arben Merkoçi, “Electrochemically reduced graphene and iridium oxide nanoparticles for inhibition-based angiotensin-converting enzyme inhibitor detection”. Biosens. Bioelectron., 88, 2017, 122–129.
- Flavio Pino, Carmen C. Mayorga-Martinez, Arben Merkoçi. “High-performance sensor based on copper oxide nanoparticles for dual detection of phenolic compounds and a pesticide”. Electrochem. Commun., 71, 2016, 33–37.
- Carmen C. Mayorga-Martinez, Maria Guix, Rossana E. Madrid, Arben Merkoçi, “Bimetallic nanowires as electrocatalyst for nonenzymatic real time impedancimetric detection of dlucose”. Chem. Comm., 48, 2012, 1686-1688.
- Everson T. S. G. da Silva , Sandrine Miserere , Lauro T. Kubota , and Arben Merkoçi .» Simple on-plastic/paper inkjet-printed solid- state Ag/AgCl pseudo-reference electrode”.Analytical Chemistry. 86, 2014, 10531–10534.
- Alejandro Zamora, Eden Morales-Narváez, Carmen C. Mayorga-Martínez, Arben Merkoçi. “Nanomaterials connected to antibodies and molecularly imprinted polymers as bio/receptors for bio/sensor applications”.Applied Materials Today, 9, 2017, 387–401